What is patent ductus arteriosus?
Patent ductus arteriosus (PDA) occurs when a blood vessel that is normal while a baby in the womb fails to close after the baby is born. The cause of the problem is not known and it affects one in 2000 babies that are born each year. It is more common in girls and much more common in premature infants. It may occur in children with otherwise normal hearts and occurs commonly in association with complex heart defects such as hypoplastic left heart syndrome, transposition of the great arteries, coarctation of the aorta, and pulmonary atresia. The information on this page applies primarily to patients with patent ductus arteriosus and otherwise normal hearts.
While a baby is in the womb, the mother provides oxygen and the baby's lungs are filled with fluid. Blood flow during this time bypasses the lungs through a blood vessel that connects the pulmonary artery (1) with the aorta (2). This blood vessel is called the ductus arteriosus (3). When it remains open after birth it is called a patent ductus arteriosus. In most babies it remains open for a short period of time after birth but 90% will be closed by 8 weeks of age. Most of the rest will close during the first year of life.
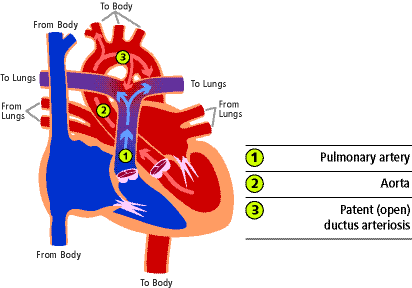
While the baby is in the womb, the fluid in the lungs causes high pressure so blood entering the pulmonary artery takes the path of least resistance bypassing the lungs and flowing out to the aorta through the ductus arteriosus. After birth, the lungs fill with oxygen so the pressure in lungs and the pulmonary artery goes down. At the same time, the umbilical cord is clamped and the pressure in the aorta increases. As a result, the pressure in the pulmonary artery is lower than the pressure in the aorta so some of the blood in the aorta flows through the ductus back to the lungs. This results in extra blood flow to the lungs. If the ductus is small, the extra blood flow is minimal but if the ductus is large, there can be a large amount of blood returning to the lungs causing a significant increased workload for the heart.
What are the effects of this problem on my child's health?
The effects of patent ductus arteriosus relate largely to the size of the ductus. Babies born very prematurely are more sensitive to the extra pulmonary blood flow so are more likely to have heart related symptoms. If the extra blood flow taxes the heart too much, symptoms of congestive heart failure develop. This is not uncommon in premature infants but is quite rare in full term infants or older children. Symptoms of congestive heart failure include rapid breathing, feeding problems, slow weight gain, low energy, and cold, clammy sweating.
If the PDA remains large, over time the extra blood flow damages the pulmonary artery and they become stiff and thickened. This condition, called pulmonary vascular disease is a very serious problem for which there is currently no effective treatment.
Children with patent ductus arteriosus are also at increased risk for subacute bacterial endocarditis (SBE). This is an infection of the heart caused by bacteria in the blood stream. It can occur after a dental or other medical procedure but can usually be prevented by a dose of antibiotic prior to the procedure. Children with small PDAs are at even greater risk for SBE than children with large PDAs. For this reason, many doctors recommend closure of even small PDAs.
Exercise recommendations: Exercise recommendations are best made by a patient's doctor so that all relevant factors can be included in the decision. In general, exercise restrictions for patients with patent ductus arteriosus are not necessary and children can participate in competitive and vigorous athletic activities.
How is this problem diagnosed?
Clinical findings: Most children with PDA do not have heart related symptoms. If the ductus is large in size, symptoms of congestive heart failure may develop. Congestive heart failure can develop at any time but more commonly presents during the first 2 to 3 months of life. The symptoms include rapid breathing, poor feeding, slow growth, and cold, clammy sweating.
Physical findings: A heart murmur is often the only clue that a child has a PDA. If the child is in congestive heart failure, there will be poor weight gain, the heart rate and breathing rate will be higher than normal, and the liver will be enlarged.
Medical tests: Medical tests that provide helpful information include an electrocardiogram, oxygen saturation test, and chest x-ray. The diagnosis is confirmed by an echocardiogram.
How is the problem treated?
As described earlier, small PDAs do not cause symptoms so generally treatment (other than SBE prophylaxis) is not needed. Many children will have spontaneous closure of the ductus during the first year of life. If the child develops congestive heart failure medications may be prescribed including digoxin and/or diuretics. These medications often control the symptoms until the child gets bigger and the PDA gets smaller or closes altogether.
If the patent ductus does not close spontaneously by one or two years of age or if there are symptoms of congestive heart failure that are not controlled by medication, closure of the defect is recommended. Closure of very small or "silent" patent ductus arteriosus may also be recommended to reduce the risk for bacterial endocarditis. Treatment options include closure via heart catheterization or surgical closure. A medicine called indomethacin is often used to close the ductus in premature infants.
Transcatheter closure of patent ductus arteriosus: Transcatheter closure has proven to be an excellent treatment option for children with patent ductus arteriosus. First reported in 1967, this procedure is done in the heart catheterization laboratory with conscious sedation and avoids the need for surgery. During the procedure, catheters (thin plastic tubes) are placed into the large blood vessels in the legs and gently guided to the heart. These catheters are used to deposit small metal coils within the ductus. The coils obstruct blood flow through the vessel, in part by stimulating the development of a blood clot at the site. This procedure achieves an excellent result in most patients. Complications are rare and include bleeding, infection, and early dislodgment of the coil. If the coil dislodges it can usually be retrieved at the time of the procedure and repositioned or replaced with a larger size coil. The procedure is done as an outpatient and children can resume all activities within 48 hours.
Surgical closure of patent ductus arteriosus: Surgical results are also excellent. Surgery is the preferred treatment for a large PDA and/or if closure is required during infancy. It is done through a small incision between the ribs on the left side. The ductus is identified and either tied off or divided. Surgical complications are rare and include hoarseness or paralyzed diaphragm, infection, bleeding, and accumulation of fluid around the lungs. Most children go home two or three days after the surgery.
Clinics
Care and services for patients with this problem are provided in the Congenital Heart, Interventional Cardiology and Cardiovascular Surgery clinics at the University of Michigan Medical Center in Ann Arbor.
What is the outlook for children with this problem?
The outlook for these patients is excellent as long as treatment is initiated soon enough to prevent pulmonary vascular obstructive disease. Possible long-term complications include coarctation (narrowing of the aorta) or recurrence of the vessel although both problems are extremely rare.
References
Lloyd TR, Fedderly R, Mendelsohn AM et al. Transcatheter occlusion of patent ductus arteriosus. Circulation 1993:88;1412-1420.
Mullins CE & Pagotto L. Patent ductus arteriosus. In Garson A, Bricker J, Fisher D & Neish S (Eds), The science and practice of pediatric cardiology. Williams & Wilkins: Baltimore, MD, 1181-1197.
Park MK. Left-to-right shunt lesions. In Pediatric cardiology for practitioners. Mosby-Year Book: St Louis, MO, 1996,142-145.
Shim D & Beekman RH. Transcatheter management of patent ductus arteriosus. Pediatr Cardiol 1998:19;67-71.
Written by: S. LeRoy RN, MSN
Reviewed September, 2012
