Childhood Central Nervous System Atypical Teratoid/Rhabdoid Tumor Treatment (PDQ®): Treatment - Patient Information [NCI]
This information is produced and provided by the National Cancer Institute (NCI). The information in this topic may have changed since it was written. For the most current information, contact the National Cancer Institute via the Internet web site at http://cancer.gov or call 1-800-4-CANCER.
General Information About Childhood Central Nervous System Atypical Teratoid / Rhabdoid Tumor
Central nervous system (CNS) atypical teratoid/rhabdoid tumor (AT/RT) is a disease in which malignant (cancer) cells form in the tissues of the brain.
Central nervous system (CNS) atypical teratoid/rhabdoid tumor (AT/RT) is a very rare, fast-growing tumor that begins in the brain and spinal cord. It usually occurs in children aged 3 years and younger, although it can occur in older children and adults.
About half of these tumors form in the cerebellum or brain stem. The cerebellum is the part of the brain that controls movement, balance, and posture. The brain stem controls breathing, heart rate, and the nerves and muscles used in seeing, hearing, walking, talking, and eating. AT/RT can also begin in other parts of the brain and spinal cord.
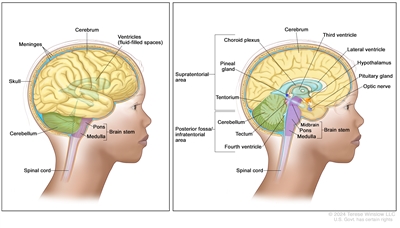
Anatomy of the brain. The supratentorial area (the upper part of the brain) contains the cerebrum, lateral ventricle and third ventricle (with cerebrospinal fluid shown in blue), choroid plexus, pineal gland, hypothalamus, pituitary gland, and optic nerve. The posterior fossa/infratentorial area (the lower back part of the brain) contains the cerebellum, tectum, fourth ventricle, and brain stem (midbrain, pons, and medulla). The tentorium separates the supratentorium from the infratentorium (right panel). The skull and meninges protect the brain and spinal cord (left panel).
This summary describes the treatment of CNS AT/RT. Treatment of metastatic brain tumors, which are tumors formed by cancer cells that begin in other parts of the body and spread to the brain, is not covered in this summary.
Brain tumors can occur in both children and adults; however, treatment for children may be different than treatment for adults. To learn about treatment for adults, see Adult Central Nervous System Tumors Treatment.
Certain genetic changes may increase the risk of AT/RT.
A risk factor is anything that increases the chance of getting a disease. Not every child with one or more of these risk factors will develop AT/RT, and it will develop in some children who don't have a known risk factor. Talk with your child's doctor if you think your child may be at risk.
AT/RT may be linked to changes in the tumor suppressor genes SMARCB1 or SMARCA4. Tumor suppressor genes make a protein that helps control how and when cells grow. Changes in the DNA of tumor suppressor genes like SMARCB1 or SMARCA4 may lead to cancer.
The changes in the SMARCB1 or SMARCA4 genes may be inherited (passed on from parents to offspring). When this gene change is inherited, tumors may form in two parts of the body at the same time (for example, in the brain and the kidney). For patients with AT/RT, genetic counseling (a discussion with a trained professional about inherited diseases and a possible need for gene testing) may be recommended.
The signs and symptoms of AT/RT are not the same in every person.
Signs and symptoms depend on the following:
- the child's age
- where the tumor has formed
Because AT/RT is fast growing, signs and symptoms may develop quickly and get worse over a period of days or weeks. Signs and symptoms may be caused by AT/RT or by other conditions. Check with your child's doctor if your child has any of the following:
- morning headache or headache that goes away after vomiting
- nausea and vomiting
- unusual sleepiness or change in activity level
- loss of balance, lack of coordination, or trouble walking
- increase in head size (in infants)
CNS AT/RT is detected (or found) with tests that examine the brain and spinal cord.
In addition to asking about your child's personal and family health history and doing a physical exam, your child's doctor may perform the following tests and procedures:
- Neurological exam: A series of questions and tests to check the brain, spinal cord, and nerve function. The exam checks a person's mental status, coordination, and ability to walk normally, and how well the muscles, senses, and reflexes work. This may also be called a neuro exam or a neurologic exam.
- MRI (magnetic resonance imaging): A procedure that uses a magnet, radio waves, and a computer to make a series of detailed pictures of areas inside the brain and spinal cord. This procedure is also called nuclear magnetic resonance imaging (NMRI).
- Lumbar puncture: A procedure used to collect cerebrospinal fluid (CSF) from the spinal column. This is done by placing a needle between two bones in the spine and into the lining around the spinal cord to remove a sample of the CSF. The sample of CSF is checked under a microscope for signs of tumor cells. The sample may also be checked for the amounts of protein and glucose. This procedure is also called an LP or spinal tap.
- SMARCB1 and SMARCA4 gene testing: A laboratory test in which a sample of blood or tissue is tested for certain changes in the SMARCB1 and SMARCA4 genes.
Childhood AT/RT is diagnosed using a biopsy, and the tumor may be removed in the same surgery.
If doctors think there might be a brain tumor, a biopsy may be done to remove a sample of tissue. For brain tumors, the biopsy can be done by removing part of the skull or making a small hole in the skull and using a needle or surgical device to remove a sample of tissue. Sometimes, when a needle is used, it is guided by a computer to remove the tissue sample. A pathologist views the tissue under a microscope to look for cancer cells. If cancer cells are found, the doctor may remove as much tumor as safely possible during the same surgery. The pathologist checks the cancer cells to find out the type of brain tumor. It is often difficult to completely remove AT/RT because of where the tumor is in the brain and because it may already have spread at the time of diagnosis. The piece of skull is usually put back in place after the procedure.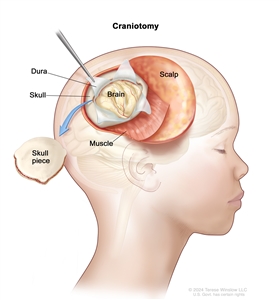
Craniotomy: An opening is made in the skull and a piece of the skull is removed to show part of the brain.
The following test may be done on the sample of tissue that is removed:
- Immunohistochemistry: A laboratory test that uses antibodies to check for certain antigens (markers) in a sample of a patient's tissue. The antibodies are usually linked to an enzyme or a fluorescent dye. After the antibodies bind to a specific antigen in the tissue sample, the enzyme or dye is activated, and the antigen can then be seen under a microscope. This type of test is used to help diagnose cancer and to help tell one type of cancer from another type of cancer.
Certain factors affect prognosis (chance of recovery) and treatment options.
The prognosis and treatment options depend on the following:
- whether there are certain inherited gene changes
- the age of the child
- the amount of tumor remaining after surgery
- whether the cancer has spread to other parts of the brain and spinal cord or to the kidney at the time of diagnosis
Stages of Childhood Central Nervous System Atypical Teratoid / Rhabdoid Tumor
There is no standard staging system for central nervous system (CNS) atypical teratoid/rhabdoid tumor (AT/RT).
The process used to find out if cancer has spread to other parts of the body is called staging. There is no standard staging system for central nervous system (CNS) atypical teratoid/rhabdoid tumor (AT/RT).
For treatment, this tumor is grouped as newly diagnosed or recurrent. Treatment depends on the following:
- the age of the child
- how much cancer remains after surgery to remove the tumor
- whether the cancer has spread to other parts of the CNS
- the results of tests and procedures done to diagnose childhood CNS AT/RT
Results from the following procedure are also used to plan treatment:
- Ultrasound exam: A procedure in which high-energy sound waves (ultrasound) are bounced off internal tissues or organs, such as the kidney, and make echoes. The echoes form a picture of body tissues called a sonogram. This procedure is done to check for tumors that may also have formed in the kidney.
Treatment Option Overview
There are different types of treatment for patients with central nervous system (CNS) atypical teratoid/rhabdoid tumor (AT/RT).
Different types of treatment are available for patients with central nervous system (CNS) atypical teratoid/rhabdoid tumor (AT/RT). Treatment for AT/RT is often within a clinical trial. A treatment clinical trial is a research study meant to help improve current treatments or obtain information on new treatments for patients with cancer.
Clinical trials are taking place in many parts of the country. Information about ongoing clinical trials is available from the NCI website. Choosing the most appropriate cancer treatment is a decision that ideally involves the patient, family, and health care team.
Children with AT/RT should have their treatment planned by a team of health care providers who are experts in treating cancer in children.
Treatment will be overseen by a pediatric oncologist, a doctor who specializes in treating children with cancer. The pediatric oncologist works with other pediatric health care providers who are experts in treating children with CNS cancer and who specialize in certain areas of medicine. Other specialists may include:
- pediatrician
- pediatric neurosurgeon
- radiation oncologist
- neurologist
- pediatric nurse specialist
- rehabilitation specialist
- psychologist
- social worker
- geneticist or genetic counselor
- fertility specialist
Childhood brain tumors may cause signs or symptoms that begin before the cancer is diagnosed and continue for months or years.
Signs or symptoms caused by the tumor may begin before diagnosis. These signs or symptoms may continue for months or years. It is important to talk with your child's doctors about signs or symptoms caused by the tumor that may continue after treatment.
The following types of treatment may be used:
Surgery
Surgery is used to diagnose and treat CNS AT/RT. To learn more about how this tumor is diagnosed, see the General Information section.
After the doctor removes all the cancer that can be seen at the time of the surgery, most patients will be given chemotherapy and possibly radiation therapy to try to kill any cancer cells that are left. Treatment given after the surgery, to lower the risk that the cancer will come back, is called adjuvant therapy.
Chemotherapy
Chemotherapy is a cancer treatment that uses drugs to stop the growth of cancer cells, either by killing the cells or by stopping them from dividing. When chemotherapy is taken by mouth or injected into a vein or muscle, the drugs enter the bloodstream and can reach tumor cells throughout the body (systemic chemotherapy). Some oral chemotherapy drugs can cross the blood-brain barrier and reach the tumor. High doses of some chemotherapy drugs given into a vein can cross the blood-brain barrier and reach the tumor. When chemotherapy is placed directly into the cerebrospinal fluid, it is called intrathecal chemotherapy. Combination chemotherapy uses more than one anticancer drug.
Childhood CNS AT/RT is treated with systemic and intrathecal chemotherapy.
Radiation therapy
Radiation therapy is a cancer treatment that uses high-energy x-rays or other types of radiation to kill cancer cells or keep them from growing. External radiation therapy uses a machine outside the body to send radiation toward the area of the body with cancer.
External radiation therapy may be given to the brain and spinal cord.
Because radiation therapy can affect growth and brain development in young children, especially children who are 3 years old or younger, the dose of radiation therapy may be lower than in older children.
High-dose chemotherapy with stem cell transplant
High doses of chemotherapy are given to kill cancer cells. Healthy cells, including blood -forming cells, are also destroyed by the cancer treatment. Stem cell transplant is a treatment to replace the blood-forming cells. Stem cells (immature blood cells) are removed from the blood or bone marrow of the patient and are frozen and stored. After the patient completes chemotherapy, the stored stem cells are thawed and given back to the patient through an infusion. These reinfused stem cells grow into (and restore) the body's blood cells.
New types of treatment are being tested in clinical trials.
This summary section describes treatments that are being studied in clinical trials. It may not mention every new treatment being studied. Information about clinical trials is available from the NCI website.
Targeted therapy
Targeted therapy uses drugs or other substances to block the action of specific enzymes, proteins, or molecules involved in the growth and spread of cancer cells. Alisertib is a type of targeted therapy being studied in the treatment of recurrent childhood CNS AT/RT.
Immunotherapy
Immunotherapy helps a person's immune system fight cancer. Biomarker tests can be used to help predict your response to certain immunotherapy drugs.
- Atezolizumab is an immunotherapy drug being studied in combination with targeted therapy (tiragolumab) to treat children who have a recurrent CNS AT/RT that has the biomarker PD-L1.
Treatment for childhood CNS AT/RT may cause side effects.
To learn about side effects that begin during treatment for cancer, see Side Effects.
Problems from cancer treatment that begin 6 months or later after treatment and continue for months or years are called late effects. Late effects of cancer treatment may include the following:
- physical problems
- changes in mood, feelings, thinking, learning, or memory
- second cancers (new types of cancer)
Some late effects may be treated or controlled. It is important to talk with your child's doctors about the effects cancer treatment can have on your child. To learn more, see Late Effects of Treatment for Childhood Cancer.
Patients may want to think about taking part in a clinical trial.
For some patients, taking part in a clinical trial may be the best treatment choice. Clinical trials are part of the cancer research process. Clinical trials are done to find out if new cancer treatments are safe and effective or better than the standard treatment.
Many of today's standard treatments for cancer are based on earlier clinical trials. Patients who take part in a clinical trial may receive the standard treatment or be among the first to receive a new treatment.
Patients who take part in clinical trials also help improve the way cancer will be treated in the future. Even when clinical trials do not lead to effective new treatments, they often answer important questions and help move research forward.
Patients can enter clinical trials before, during, or after starting their cancer treatment.
Some clinical trials only include patients who have not yet received treatment. Other trials test treatments for patients whose cancer has not gotten better. There are also clinical trials that test new ways to stop cancer from recurring (coming back) or reduce the side effects of cancer treatment.
Clinical trials are taking place in many parts of the country. Information about clinical trials supported by NCI can be found on NCI's clinical trials search webpage. Clinical trials supported by other organizations can be found on the ClinicalTrials.gov website.
Follow-up tests may be needed.
Some of the tests that were done to diagnose the cancer may be repeated. Some tests will be repeated to see how well the treatment is working. Decisions about whether to continue, change, or stop treatment may be based on the results of these tests.
Some of the tests will continue to be done from time to time after treatment has ended. The results of these tests can show if your child's condition has changed or if the cancer has recurred (come back). These tests are sometimes called follow-up tests or check ups.
Treatment of Childhood Central Nervous System Atypical Teratoid / Rhabdoid Tumor
For information about the treatments listed below, see the Treatment Option Overview section.
There is no standard treatment for children with newly diagnosed central nervous system (CNS) atypical teratoid/rhabdoid tumor (AT/RT). Because AT/RT is fast-growing, a combination of treatments is usually given.
After surgery to remove the tumor, treatments for AT/RT may include combinations of the following:
- chemotherapy
- radiation therapy
- high-dose chemotherapy with stem cell transplant
Use our clinical trial search to find NCI-supported cancer clinical trials that are accepting patients. You can search for trials based on the type of cancer, the age of the patient, and where the trials are being done. General information about clinical trials is also available.
Treatment of Recurrent Childhood Central Nervous System Atypical Teratoid / Rhabdoid Tumor
For information about the treatments listed below, see the Treatment Option Overview section.
There is no standard treatment for children with recurrent childhood central nervous system atypical teratoid/rhabdoid tumor. Treatment may include the following:
- radiation therapy
- palliative treatment to improve quality of life
- a clinical trial of targeted therapy (alisertib)
- a clinical trial of targeted therapy (tiragolumab) given with immunotherapy (atezolizumab)
Use our clinical trial search to find NCI-supported cancer clinical trials that are accepting patients. You can search for trials based on the type of cancer, the age of the patient, and where the trials are being done. General information about clinical trials is also available.
To Learn More about Childhood Central Nervous System Atypical Teratoid / Rhabdoid Tumor and Other Childhood Brain Tumors
For more information about childhood central nervous system atypical teratoid/rhabdoid tumor and other childhood brain tumors, see the following:
- Pediatric Brain Tumor Consortium (PBTC)
- Targeted Therapy to Treat Cancer
For more childhood cancer information and other general cancer resources, see the following:
- About Cancer
- Childhood Cancers
- CureSearch for Children's Cancer
- Late Effects of Treatment for Childhood Cancer
- Adolescents and Young Adults with Cancer
- Children with Cancer: A Guide for Parents
- Cancer in Children and Adolescents
- Staging
- Coping with Cancer
- Questions to Ask Your Doctor about Cancer
- For Survivors and Caregivers
About This PDQ Summary
About PDQ
Physician Data Query (PDQ) is the National Cancer Institute's (NCI's) comprehensive cancer information database. The PDQ database contains summaries of the latest published information on cancer prevention, detection, genetics, treatment, supportive care, and complementary and alternative medicine. Most summaries come in two versions. The health professional versions have detailed information written in technical language. The patient versions are written in easy-to-understand, nontechnical language. Both versions have cancer information that is accurate and up to date and most versions are also available in Spanish.
PDQ is a service of the NCI. The NCI is part of the National Institutes of Health (NIH). NIH is the federal government's center of biomedical research. The PDQ summaries are based on an independent review of the medical literature. They are not policy statements of the NCI or the NIH.
Purpose of This Summary
This PDQ cancer information summary has current information about the treatment of childhood central nervous system atypical teratoid and rhabdoid tumor. It is meant to inform and help patients, families, and caregivers. It does not give formal guidelines or recommendations for making decisions about health care.
Reviewers and Updates
Editorial Boards write the PDQ cancer information summaries and keep them up to date. These Boards are made up of experts in cancer treatment and other specialties related to cancer. The summaries are reviewed regularly and changes are made when there is new information. The date on each summary ("Updated") is the date of the most recent change.
The information in this patient summary was taken from the health professional version, which is reviewed regularly and updated as needed, by the PDQ Pediatric Treatment Editorial Board.
Clinical Trial Information
A clinical trial is a study to answer a scientific question, such as whether one treatment is better than another. Trials are based on past studies and what has been learned in the laboratory. Each trial answers certain scientific questions in order to find new and better ways to help cancer patients. During treatment clinical trials, information is collected about the effects of a new treatment and how well it works. If a clinical trial shows that a new treatment is better than one currently being used, the new treatment may become "standard." Patients may want to think about taking part in a clinical trial. Some clinical trials are open only to patients who have not started treatment.
Clinical trials can be found online at NCI's website. For more information, call the Cancer Information Service (CIS), NCI's contact center, at 1-800-4-CANCER (1-800-422-6237).
Permission to Use This Summary
PDQ is a registered trademark. The content of PDQ documents can be used freely as text. It cannot be identified as an NCI PDQ cancer information summary unless the whole summary is shown and it is updated regularly. However, a user would be allowed to write a sentence such as "NCI's PDQ cancer information summary about breast cancer prevention states the risks in the following way: [include excerpt from the summary]."
The best way to cite this PDQ summary is:
PDQ® Pediatric Treatment Editorial Board. PDQ Childhood Central Nervous System Atypical Teratoid/Rhabdoid Tumor Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/brain/patient/child-cns-atrt-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389341]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use in the PDQ summaries only. If you want to use an image from a PDQ summary and you are not using the whole summary, you must get permission from the owner. It cannot be given by the National Cancer Institute. Information about using the images in this summary, along with many other images related to cancer can be found in Visuals Online. Visuals Online is a collection of more than 3,000 scientific images.
Disclaimer
The information in these summaries should not be used to make decisions about insurance reimbursement. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website's E-mail Us.
Last Revised: 2023-12-15
If you want to know more about cancer and how it is treated, or if you wish to know about clinical trials for your type of cancer, you can call the NCI's Cancer Information Service at 1-800-422-6237, toll free. A trained information specialist can talk with you and answer your questions.
Topic Contents
- General Information About Childhood Central Nervous System Atypical Teratoid / Rhabdoid Tumor
- Stages of Childhood Central Nervous System Atypical Teratoid / Rhabdoid Tumor
- Treatment Option Overview
- Treatment of Childhood Central Nervous System Atypical Teratoid / Rhabdoid Tumor
- Treatment of Recurrent Childhood Central Nervous System Atypical Teratoid / Rhabdoid Tumor
- To Learn More about Childhood Central Nervous System Atypical Teratoid / Rhabdoid Tumor and Other Childhood Brain Tumors
- About This PDQ Summary
This information does not replace the advice of a doctor. Healthwise, Incorporated, disclaims any warranty or liability for your use of this information. Your use of this information means that you agree to the Terms of Use. Learn how we develop our content.
