Childhood Brain Stem Glioma Treatment (PDQ®): Treatment - Health Professional Information [NCI]
This information is produced and provided by the National Cancer Institute (NCI). The information in this topic may have changed since it was written. For the most current information, contact the National Cancer Institute via the Internet web site at http://cancer.gov or call 1-800-4-CANCER.
General Information About Childhood Brain Stem Glioma
Primary brain tumors, including brain stem gliomas, are a diverse group of diseases that together constitute the most common solid tumor of childhood. Immunohistochemical analysis, cytogenetic and molecular genetic findings, and measures of mitotic activity are increasingly used in tumor diagnosis and classification. Brain tumors are classified according to histology, but tumor location and extent of spread are important factors that affect treatment and prognosis.
The PDQ childhood brain tumor treatment summaries are organized primarily according to the World Health Organization (WHO) classification of nervous system tumors.[1] For a full description of the classification of nervous system tumors and a link to the corresponding treatment summary for each type of brain tumor, refer to the PDQ summary on Childhood Brain and Spinal Cord Tumors Treatment Overview.
The term brain stem glioma is a generic description that refers to any tumor of glial origin arising in the brain stem, inclusive of the midbrain, pons, and medulla. While other histologies (e.g., ganglioglioma) can occur in the brain stem, the following two histologies predominate:
- Diffuse astrocytomas centered in the pons, also called diffuse intrinsic pontine glioma (DIPG).
- Pilocytic astrocytomas, which occur throughout the brain stem.
Incidence
Approximately 300 to 400 pediatric brain stem tumors are diagnosed each year in the United States. DIPG accounts for approximately 75% to 80% of pediatric brain stem tumors.[2] Most children with DIPG are diagnosed between the ages of 5 and 10 years. Focal pilocytic astrocytomas in the brain stem occur less frequently.[3]
Anatomy
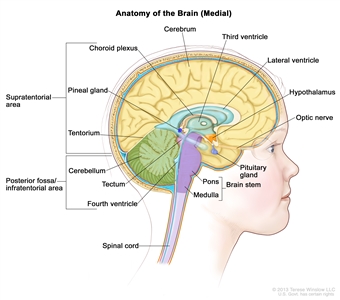
Anatomy of the inside of the brain, showing the pineal and pituitary glands, optic nerve, ventricles (with cerebrospinal fluid shown in blue), and other parts of the brain. The posterior fossa is the region below the tentorium, which separates the cortex from the cerebellum and essentially denotes the region containing the brain stem, cerebellum, and fourth ventricle.
Clinical Features
Children with DIPG may present with the following classic triad of symptoms; however, children may present with only one or two of these symptoms at diagnosis:
- Cranial neuropathies, particularly abducens paresis.
- Long tract signs.
- Ataxia.
Obstructive hydrocephalus caused by expansion of the pons can also be a presenting symptom. Nonspecific symptoms may also occur, including behavioral changes and decreased school performance.
The presentation of focal pilocytic astrocytomas in the brain stem depends on the tumor location. Common presenting symptoms include the following:[3]
- Raised intracranial pressure with associated hydrocephalus.
- Unilateral hemiparesis.
- Unilateral cranial neuropathies.
- Ataxia.
Diagnosis
Primary tumors of the brain stem are most often diagnosed on the basis of clinical findings and on neuroimaging studies using magnetic resonance imaging (MRI), as follows:[4]
- DIPG. A presumptive diagnosis of DIPG based on classic imaging features, in the absence of a histologic diagnosis, has been routinely employed. Increasingly however, histologic confirmation is obtained for both entry into research studies and molecular characterization of the tumor.[5] New approaches with stereotactic needle biopsy may make biopsy safer.[6,7,8,9] Biopsy is recommended for pontine tumors when the diagnosis is uncertain based on imaging findings.
- Non-DIPG brain stem tumors. Biopsy or resection is generally indicated for non-DIPG brain stem tumors.
Children with neurofibromatosis type 1 (NF1) are at an increased risk of developing brain stem gliomas, often pilocytic astrocytomas and not DIPGs. They may present with a long history of symptoms or be identified by screening tests.
Prognosis and Prognostic Factors
The median survival for children with DIPGs is less than 1 year, although about 10% of children will survive longer than 2 years.[10,11] In contrast, focal astrocytomas (e.g., pilocytic astrocytomas) have a markedly improved prognosis, with 5-year overall survival exceeding 90%.[3]
Prognostic factors include the following:
- Histology/grade of the tumor: Astrocytic tumors predominate in the brain stem. WHO grade 1 tumors (e.g., pilocytic astrocytomas and gangliogliomas) have a favorable prognosis and can arise throughout the brain stem, including the tectum of the midbrain, focally within the pons, or at the cervicomedullary junction where they are often exophytic. Low-grade diffuse astrocytomas (WHO grade 2) occurring outside the pons in other brain stem locations tend to be tumors with a more favorable prognosis.[12]
DIPGs are diffuse astrocytomas that, when biopsied at diagnosis, can range from diffuse astrocytomas (WHO grade 2) to glioblastomas (WHO grade 4). At postmortem evaluation, DIPGs are also generally anaplastic astrocytomas (WHO grade 3) or glioblastomas (WHO grade 4) by morphological criteria, although WHO grade 2 regions can also be identified.[13,14,15,16,17]
Approximately 80% of DIPGs, regardless of histologic grade, demonstrate a histone H3.3 or H3.1 mutation and are now classified by the WHO as diffuse midline gliomas, H3 K27M-mutant (refer to the Cytogenetic Characteristics of Diffuse Intrinsic Pontine Gliomas section of this summary for more information). All diffuse midline gliomas, H3 K27M-mutant, are WHO grade 4, regardless of histologic grade, reflecting the poor prognosis of children with this diagnosis.
- Age at diagnosis: Approximately 4% of children with DIPGs are diagnosed when younger than 3 years. The prognosis of these children is more favorable than that of older children, with 28% of younger children alive at 2 years compared with 8% of children aged 3 to 10 years at diagnosis and 14% of children older than 10 years at diagnosis. The more favorable prognosis for young children may reflect the presence of different biological characteristics in different age groups.[10,18]
- NF1: Children with NF1 and brain stem gliomas may have a better prognosis than other patients who have intrinsic lesions.[19,20]
- Clinical and imaging features present at diagnosis: For children with DIPGs, features associated with surviving less than 2 years include the presence at diagnosis of cranial nerve palsies, ring enhancement, necrosis, and extrapontine extension.[10] Two-year survival for patients with these characteristics is less than 10%.
- Duration of symptoms at diagnosis: Longer duration of symptoms is associated with a more favorable prognosis. Two-year survival rates range from 7% for patients with duration of symptoms less than 6 months to 29% for patients with duration of symptoms of 24 months or longer.[10]
- Histone mutations: Patients with H3.1 K27M mutations have a longer median survival (15 months) than do patients with H3.3 K27M mutations (10.4 months) or patients without a histone mutation (10.5 months).[10]
Follow-up After Treatment
For children with brain stem tumors and anticipated long-term survival, standard follow-up tends to include interval clinical assessments and periodic imaging with MRI. The required duration of follow-up with MRI varies; it largely depends on the presence or absence of residual imaging abnormalities after treatment, and the original histology of the tumor.
References:
- Louis DN, Ohgaki H, Wiestler OD: WHO Classification of Tumours of the Central Nervous System. 4th rev.ed. IARC Press, 2016.
- Warren KE: Diffuse intrinsic pontine glioma: poised for progress. Front Oncol 2: 205, 2012.
- Klimo P, Pai Panandiker AS, Thompson CJ, et al.: Management and outcome of focal low-grade brainstem tumors in pediatric patients: the St. Jude experience. J Neurosurg Pediatr 11 (3): 274-81, 2013.
- Liu AK, Brandon J, Foreman NK, et al.: Conventional MRI at presentation does not predict clinical response to radiation therapy in children with diffuse pontine glioma. Pediatr Radiol 39 (12): 1317-20, 2009.
- Walker DA, Liu J, Kieran M, et al.: A multi-disciplinary consensus statement concerning surgical approaches to low-grade, high-grade astrocytomas and diffuse intrinsic pontine gliomas in childhood (CPN Paris 2011) using the Delphi method. Neuro Oncol 15 (4): 462-8, 2013.
- Cage TA, Samagh SP, Mueller S, et al.: Feasibility, safety, and indications for surgical biopsy of intrinsic brainstem tumors in children. Childs Nerv Syst 29 (8): 1313-9, 2013.
- Grill J, Puget S, Andreiuolo F, et al.: Critical oncogenic mutations in newly diagnosed pediatric diffuse intrinsic pontine glioma. Pediatr Blood Cancer 58 (4): 489-91, 2012.
- Puget S, Beccaria K, Blauwblomme T, et al.: Biopsy in a series of 130 pediatric diffuse intrinsic Pontine gliomas. Childs Nerv Syst 31 (10): 1773-80, 2015.
- Gupta N, Goumnerova LC, Manley P, et al.: Prospective feasibility and safety assessment of surgical biopsy for patients with newly diagnosed diffuse intrinsic pontine glioma. Neuro Oncol 20 (11): 1547-1555, 2018.
- Hoffman LM, Veldhuijzen van Zanten SEM, Colditz N, et al.: Clinical, Radiologic, Pathologic, and Molecular Characteristics of Long-Term Survivors of Diffuse Intrinsic Pontine Glioma (DIPG): A Collaborative Report From the International and European Society for Pediatric Oncology DIPG Registries. J Clin Oncol 36 (19): 1963-1972, 2018.
- Cohen KJ, Pollack IF, Zhou T, et al.: Temozolomide in the treatment of high-grade gliomas in children: a report from the Children's Oncology Group. Neuro Oncol 13 (3): 317-23, 2011.
- McAbee JH, Modica J, Thompson CJ, et al.: Cervicomedullary tumors in children. J Neurosurg Pediatr 16 (4): 357-66, 2015.
- Ballester LY, Wang Z, Shandilya S, et al.: Morphologic characteristics and immunohistochemical profile of diffuse intrinsic pontine gliomas. Am J Surg Pathol 37 (9): 1357-64, 2013.
- Wu G, Diaz AK, Paugh BS, et al.: The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat Genet 46 (5): 444-50, 2014.
- Taylor KR, Mackay A, Truffaux N, et al.: Recurrent activating ACVR1 mutations in diffuse intrinsic pontine glioma. Nat Genet 46 (5): 457-61, 2014.
- Buczkowicz P, Hoeman C, Rakopoulos P, et al.: Genomic analysis of diffuse intrinsic pontine gliomas identifies three molecular subgroups and recurrent activating ACVR1 mutations. Nat Genet 46 (5): 451-6, 2014.
- Hoffman LM, DeWire M, Ryall S, et al.: Spatial genomic heterogeneity in diffuse intrinsic pontine and midline high-grade glioma: implications for diagnostic biopsy and targeted therapeutics. Acta Neuropathol Commun 4: 1, 2016.
- Broniscer A, Laningham FH, Sanders RP, et al.: Young age may predict a better outcome for children with diffuse pontine glioma. Cancer 113 (3): 566-72, 2008.
- Pascual-Castroviejo I, Pascual-Pascual SI, Viaño J, et al.: Posterior fossa tumors in children with neurofibromatosis type 1 (NF1). Childs Nerv Syst 26 (11): 1599-603, 2010.
- Albers AC, Gutmann DH: Gliomas in patients with neurofibromatosis type 1. Expert Rev Neurother 9 (4): 535-9, 2009.
Molecular Features of Childhood Brain Stem Glioma
Cytogenetic Characteristics of Diffuse Intrinsic Pontine Gliomas (DIPGs)
Genomics of DIPGs
The genomic characteristics of DIPGs appear to differ from those of many other pediatric high-grade gliomas of the cerebrum and from those of adult high-grade gliomas.[1] The molecular and clinical characteristics of DIPGs align with those of other midline high-grade gliomas, with a specific H3 K27M mutation in histone H3.3 (H3F3A) or H3.1 (HIST1H3B and HIST1H3C), which led the World Health Organization to group these tumors together into a single entity, called diffuse midline glioma, H3 K27M-mutant.[2]
In one report of 64 children with thalamic tumors, 50% of high-grade gliomas (11 of 22) had an H3 K27M mutation, and approximately 10% of tumors with low-grade morphological characteristics (5 of 42) had an H3 K27M mutation. Five-year overall survival (OS) was only 6% (1 of 16).[3] In another study that included 202 children with glioblastoma, 68 of the tumors were midline (primarily thalamic) and had an H3 K27M mutation. Five-year OS for this group was only 5%, which was significantly inferior to the survival rates of the remaining patients in the study.[4]
A number of chromosomal and genomic abnormalities have been reported for DIPG, including the following:
- Histone H3 genes: Approximately 80% of DIPG tumors have a mutation in a specific amino acid in the histone H3.3 (H3F3A) or H3.1 (HIST1H3B and HIST1H3C) genes.[5,6,7,8,9] This H3 K27M mutation is observed in pediatric high-grade gliomas at other midline locations but is uncommon in cerebral pediatric high-grade gliomas and in adult high-grade gliomas.[5,6,7,8,9,10]
An autopsy study that examined multiple tumor sites (primary, contiguous, and metastatic) in seven DIPG patients found that the H3 K27M mutation was invariably present, supporting its role as a driver mutation for DIPG.[11]
Patients with H3.1 K27M mutations have a longer median survival (15 months) than do patients with H3.3 K27M mutations (10.4 months).[12]
- ACVR1 gene: Approximately 20% of DIPG cases have activating mutations in the ACVR1 gene, with most occurring concurrently with H3.3 mutations.[6,7,8,9]
- Receptor tyrosine kinase amplification: PDGFRA amplification occurs in approximately 30% of cases, with lower rates of amplification observed for some other receptor tyrosine kinase genes (e.g., MET and IGF1R).[13,14]
An autopsy study that examined multiple tumor sites (primary, contiguous, and metastatic) in seven DIPG patients found that PDGFRA amplification was variably present across these sites, suggesting that this change is a secondary genomic alteration in DIPG.[11]
- TP53 deletion: DIPG tumors commonly show deletion of the TP53 gene on chromosome 17p.[14] Additionally, TP53 is commonly mutated in DIPG tumors, particularly those with histone H3 gene mutations.[6,7,8,9,15] Aneuploidy is commonly observed in cases with TP53 mutations.[9]
The gene expression profile of DIPG differs from that of non–brain stem pediatric high-grade gliomas, further supporting a distinctive biology for this subset of pediatric gliomas.[14] Pediatric H3 K27M-mutant tumors rarely show O-6-methylguanine-DNA methyltransferase (MGMT) promoter methylation,[4] which explains the lack of efficacy of temozolomide when it was tested in patients with DIPG.[16]
(Refer to the Genomic Alterations section in the PDQ summary on Childhood Astrocytomas Treatment for more information about the genetics of low-grade gliomas.)
References:
- Jones C, Karajannis MA, Jones DTW, et al.: Pediatric high-grade glioma: biologically and clinically in need of new thinking. Neuro Oncol 19 (2): 153-161, 2017.
- Louis DN, Perry A, Reifenberger G, et al.: The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 131 (6): 803-20, 2016.
- Ryall S, Krishnatry R, Arnoldo A, et al.: Targeted detection of genetic alterations reveal the prognostic impact of H3K27M and MAPK pathway aberrations in paediatric thalamic glioma. Acta Neuropathol Commun 4 (1): 93, 2016.
- Korshunov A, Ryzhova M, Hovestadt V, et al.: Integrated analysis of pediatric glioblastoma reveals a subset of biologically favorable tumors with associated molecular prognostic markers. Acta Neuropathol 129 (5): 669-78, 2015.
- Wu G, Broniscer A, McEachron TA, et al.: Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet 44 (3): 251-3, 2012.
- Wu G, Diaz AK, Paugh BS, et al.: The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat Genet 46 (5): 444-50, 2014.
- Fontebasso AM, Papillon-Cavanagh S, Schwartzentruber J, et al.: Recurrent somatic mutations in ACVR1 in pediatric midline high-grade astrocytoma. Nat Genet 46 (5): 462-6, 2014.
- Taylor KR, Mackay A, Truffaux N, et al.: Recurrent activating ACVR1 mutations in diffuse intrinsic pontine glioma. Nat Genet 46 (5): 457-61, 2014.
- Buczkowicz P, Hoeman C, Rakopoulos P, et al.: Genomic analysis of diffuse intrinsic pontine gliomas identifies three molecular subgroups and recurrent activating ACVR1 mutations. Nat Genet 46 (5): 451-6, 2014.
- Schwartzentruber J, Korshunov A, Liu XY, et al.: Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature 482 (7384): 226-31, 2012.
- Hoffman LM, DeWire M, Ryall S, et al.: Spatial genomic heterogeneity in diffuse intrinsic pontine and midline high-grade glioma: implications for diagnostic biopsy and targeted therapeutics. Acta Neuropathol Commun 4: 1, 2016.
- Hoffman LM, Veldhuijzen van Zanten SEM, Colditz N, et al.: Clinical, Radiologic, Pathologic, and Molecular Characteristics of Long-Term Survivors of Diffuse Intrinsic Pontine Glioma (DIPG): A Collaborative Report From the International and European Society for Pediatric Oncology DIPG Registries. J Clin Oncol 36 (19): 1963-1972, 2018.
- Zarghooni M, Bartels U, Lee E, et al.: Whole-genome profiling of pediatric diffuse intrinsic pontine gliomas highlights platelet-derived growth factor receptor alpha and poly (ADP-ribose) polymerase as potential therapeutic targets. J Clin Oncol 28 (8): 1337-44, 2010.
- Paugh BS, Broniscer A, Qu C, et al.: Genome-wide analyses identify recurrent amplifications of receptor tyrosine kinases and cell-cycle regulatory genes in diffuse intrinsic pontine glioma. J Clin Oncol 29 (30): 3999-4006, 2011.
- Khuong-Quang DA, Buczkowicz P, Rakopoulos P, et al.: K27M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol 124 (3): 439-47, 2012.
- Cohen KJ, Heideman RL, Zhou T, et al.: Temozolomide in the treatment of children with newly diagnosed diffuse intrinsic pontine gliomas: a report from the Children's Oncology Group. Neuro Oncol 13 (4): 410-6, 2011.
Stage Information for Childhood Brain Stem Glioma
There is no generally applied staging system for childhood brain stem glioma.
Brain stem gliomas are classified according to the following factors:
- Location.
- Radiographic appearance.
- Histology (when obtained).
Brain stem gliomas may occur in the pons, midbrain, tectum, dorsum of the medulla at the cervicomedullary junction, or in multiple regions of the brain stem. The tumor may contiguously involve the cerebellar peduncles, cerebellum, the cervical spinal cord, and/or thalamus.[1,2]
Spread of diffuse intrinsic pontine gliomas (DIPGs), noted clinically, is usually contiguous, with metastasis via the subarachnoid space. Such dissemination may occur before local progression but usually occurs simultaneously with or after primary disease progression.[3] However, subclinically, more widespread dissemination with extension to the brain stem, thalamus, cerebrum, and supratentorial leptomeninges is noted at autopsy.[4]
References:
- Laigle-Donadey F, Doz F, Delattre JY: Brainstem gliomas in children and adults. Curr Opin Oncol 20 (6): 662-7, 2008.
- Khatua S, Moore KR, Vats TS, et al.: Diffuse intrinsic pontine glioma-current status and future strategies. Childs Nerv Syst 27 (9): 1391-7, 2011.
- Sethi R, Allen J, Donahue B, et al.: Prospective neuraxis MRI surveillance reveals a high risk of leptomeningeal dissemination in diffuse intrinsic pontine glioma. J Neurooncol 102 (1): 121-7, 2011.
- Caretti V, Bugiani M, Freret M, et al.: Subventricular spread of diffuse intrinsic pontine glioma. Acta Neuropathol 128 (4): 605-7, 2014.
Treatment Option Overview for Childhood Brain Stem Glioma
Dramatic improvements in survival have been achieved for children and adolescents with cancer. Between 1975 and 2010, childhood cancer mortality decreased by more than 50%.[1] Many of the improvements in survival in childhood cancer have been made as a result of clinical trials that have attempted to improve on the best available, accepted therapy. Clinical trials in pediatrics are designed to compare new therapy with therapy that is currently accepted as standard. This comparison may be done in a randomized study of two treatment arms or by evaluating a single new treatment and comparing the results with those that were previously obtained with existing therapy.
Because of the relative rarity of cancer in children, all patients with brain tumors should be considered for entry into a clinical trial. To determine and implement optimum treatment, planning by a multidisciplinary team of cancer specialists who have experience treating childhood brain tumors is required. Radiation therapy (including 3-dimensional conformal radiation therapy) of pediatric brain tumors is technically very demanding and should be carried out in centers that have experience in that area to ensure optimal results.
Table 1 describes the standard treatment options for newly diagnosed and progressive or recurrent childhood brain stem gliomas.
| Treatment Group | Standard Treatment Options | |
|---|---|---|
| Newly diagnosed childhood brain stem gliomas: | ||
| Diffuse intrinsic pontine gliomas | Radiation therapy | |
| Focal brain stem gliomas | Surgical resection (with or without chemotherapy and/or radiation therapy) | |
| Observation (with or without cerebrospinal fluid diversion) | ||
| Radiation therapy, chemotherapy, or alternative approaches for unresectable tumors | ||
| Progressive/recurrent childhood brain stem gliomas: | ||
| Diffuse intrinsic pontine gliomas | There is no standard treatment | |
| Focal brain stem gliomas | Surgery | |
| Radiation therapy | ||
| Chemotherapy | ||
References:
- Smith MA, Altekruse SF, Adamson PC, et al.: Declining childhood and adolescent cancer mortality. Cancer 120 (16): 2497-506, 2014.
Newly Diagnosed Childhood Brain Stem Glioma Treatment
Standard Treatment Options for Newly Diagnosed Childhood Diffuse Intrinsic Pontine Gliomas (DIPGs)
Standard treatment options for newly diagnosed childhood DIPGs include the following:
- Radiation therapy.
While numerous clinical trials are available for children with newly diagnosed DIPGs, the utility of any therapy besides radiation therapy in the treatment of patients with newly diagnosed DIPG remains unproven.[1,2,3,4,5,6]; [7,8][Level of evidence: 2A]; [9][Level of evidence: 3iiiA]
No chemotherapeutic (including neoadjuvant, concurrent, postradiation chemotherapy) or immunotherapy strategy, when added to radiation therapy, has led to long-term survival for children with DIPGs.[10,11,12]; [13][Level of evidence: 2A] This includes therapy using high-dose, marrow-ablative chemotherapy with autologous hematopoietic stem cell rescue, which has been shown to be ineffective in extending survival.[14] However, similar to the treatment of other brain tumors, radiation therapy is generally omitted for infants with DIPGs, and chemotherapy-only approaches are utilized. Published data supporting the utility of this approach are lacking.
Radiation therapy
Standard treatment for children with DIPGs is radiation therapy to involved areas. The conventional dose of radiation ranges between 54 Gy and 60 Gy given locally to the primary tumor site in single daily fractions. Such treatment will result in transient benefit for most patients, but more than 90% of patients will die within 18 months of diagnosis.[15]
Radiation-induced changes may occur a few months after the completion of radiation therapy and may mimic tumor progression. When considering the efficacy of additional treatment, care needs to be taken to separate radiation-induced change from progressive disease.[16]
Research studies that evaluated the efficacy of hyperfractionated and hypofractionated radiation therapy and radiosensitizers have not demonstrated improved outcomes using these radiation techniques.
- Hyperfractionated (twice daily) radiation therapy. This technique has been used to deliver a higher dose of radiation, and studies using doses as high as 78 Gy have been completed. Evidence demonstrates that these increased radiation therapy doses do not improve the duration or rate of survival for patients with DIPGs, whether given alone [1,17] or in combination with chemotherapy.[3]
- Hypofractionated radiation therapy. This technique results in survival rates comparable to conventional fractionated radiation therapy techniques, possibly with less treatment burden.[18]; [19][Level of evidence: 1iiA]; [15,20][Level of evidence: 2A]
- Radiosensitizers. Studies evaluating the efficacy of various radiosensitizers as a means for enhancing the therapeutic effect of radiation therapy have been completed but have failed to show any significant improvement in outcome.[1,3,4,5,21,22]
Treatment options under clinical evaluation for newly diagnosed childhood DIPGs
Early-phase therapeutic trials may be available for selected patients. These trials may be available via the Children's Oncology Group (COG), the Pediatric Brain Tumor Consortium (PBTC), or other entities. Information about National Cancer Institute (NCI)–supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
Standard Treatment Options for Newly Diagnosed Childhood Focal Brain Stem Gliomas
Standard treatment options for newly diagnosed childhood focal brain stem gliomas include the following:
- Surgical resection (with or without chemotherapy and/or radiation therapy).
- Observation (with or without cerebrospinal fluid diversion).
- Radiation therapy, chemotherapy, or alternative approaches for unresectable tumors.
Surgical resection (with or without chemotherapy and/or radiation therapy)
In general, maximal surgical resection is attempted.[23]
Patients with residual tumor may be candidates for additional therapy. Treatment options include chemotherapy and/or radiation therapy.
Observation (with or without cerebrospinal fluid diversion)
Patients with small tectal lesions and hydrocephalus but no other neurological deficits may be treated with cerebrospinal fluid diversion alone and undergo monitoring with sequential neuroradiographic studies unless there is evidence of progressive disease.[24]
For patients with neurofibromatosis type 1, a period of observation may be indicated before instituting any treatment.[25] Brain stem gliomas in these children may be indolent and may require no specific treatment for years.[26]
Radiation therapy, chemotherapy, or alternative approaches for unresectable tumors
In selected circumstances, adjuvant therapy in the form of radiation therapy or chemotherapy can be considered in a child with a newly diagnosed focal brain stem glioma.[27,28][Level of evidence: 3iDi] Decisions regarding the need for such therapy depend on the age of the child, the extent of resection obtained, and associated neurologic deficits.
Stereotactic iodine I 125 brachytherapy, with or without adjuvant chemotherapy, is a rarely used alternative approach that has proven beneficial in select cases.[29]
Treatment options under clinical evaluation for newly diagnosed childhood focal brain stem gliomas
Early-phase therapeutic trials may be available for selected patients. These trials may be available via the COG, the PBTC, or other entities. Information about NCI-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- NCT02684058 (Phase II Pediatric Study With Dabrafenib in Combination With Trametinib in Patients With High-Grade Gliomas and Low-Grade Gliomas): A study to investigate the activity of dabrafenib in combination with trametinib in children and adolescent patients with BRAF V600 mutation–positive low-grade gliomas or relapsed or refractory high-grade gliomas.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References:
- Mandell LR, Kadota R, Freeman C, et al.: There is no role for hyperfractionated radiotherapy in the management of children with newly diagnosed diffuse intrinsic brainstem tumors: results of a Pediatric Oncology Group phase III trial comparing conventional vs. hyperfractionated radiotherapy. Int J Radiat Oncol Biol Phys 43 (5): 959-64, 1999.
- Jennings MT, Sposto R, Boyett JM, et al.: Preradiation chemotherapy in primary high-risk brainstem tumors: phase II study CCG-9941 of the Children's Cancer Group. J Clin Oncol 20 (16): 3431-7, 2002.
- Allen J, Siffert J, Donahue B, et al.: A phase I/II study of carboplatin combined with hyperfractionated radiotherapy for brainstem gliomas. Cancer 86 (6): 1064-9, 1999.
- Broniscer A, Leite CC, Lanchote VL, et al.: Radiation therapy and high-dose tamoxifen in the treatment of patients with diffuse brainstem gliomas: results of a Brazilian cooperative study. Brainstem Glioma Cooperative Group. J Clin Oncol 18 (6): 1246-53, 2000.
- Doz F, Neuenschwander S, Bouffet E, et al.: Carboplatin before and during radiation therapy for the treatment of malignant brain stem tumours: a study by the Société Française d'Oncologie Pédiatrique. Eur J Cancer 38 (6): 815-9, 2002.
- Wolff JE, Westphal S, Mölenkamp G, et al.: Treatment of paediatric pontine glioma with oral trophosphamide and etoposide. Br J Cancer 87 (9): 945-9, 2002.
- Korones DN, Fisher PG, Kretschmar C, et al.: Treatment of children with diffuse intrinsic brain stem glioma with radiotherapy, vincristine and oral VP-16: a Children's Oncology Group phase II study. Pediatr Blood Cancer 50 (2): 227-30, 2008.
- Cohen KJ, Heideman RL, Zhou T, et al.: Temozolomide in the treatment of children with newly diagnosed diffuse intrinsic pontine gliomas: a report from the Children's Oncology Group. Neuro Oncol 13 (4): 410-6, 2011.
- Jalali R, Raut N, Arora B, et al.: Prospective evaluation of radiotherapy with concurrent and adjuvant temozolomide in children with newly diagnosed diffuse intrinsic pontine glioma. Int J Radiat Oncol Biol Phys 77 (1): 113-8, 2010.
- Frappaz D, Schell M, Thiesse P, et al.: Preradiation chemotherapy may improve survival in pediatric diffuse intrinsic brainstem gliomas: final results of BSG 98 prospective trial. Neuro Oncol 10 (4): 599-607, 2008.
- Frazier JL, Lee J, Thomale UW, et al.: Treatment of diffuse intrinsic brainstem gliomas: failed approaches and future strategies. J Neurosurg Pediatr 3 (4): 259-69, 2009.
- Hargrave D, Bartels U, Bouffet E: Diffuse brainstem glioma in children: critical review of clinical trials. Lancet Oncol 7 (3): 241-8, 2006.
- Warren K, Bent R, Wolters PL, et al.: A phase 2 study of pegylated interferon α-2b (PEG-Intron(®)) in children with diffuse intrinsic pontine glioma. Cancer 118 (14): 3607-13, 2012.
- Bouffet E, Raquin M, Doz F, et al.: Radiotherapy followed by high dose busulfan and thiotepa: a prospective assessment of high dose chemotherapy in children with diffuse pontine gliomas. Cancer 88 (3): 685-92, 2000.
- Janssens GO, Jansen MH, Lauwers SJ, et al.: Hypofractionation vs conventional radiation therapy for newly diagnosed diffuse intrinsic pontine glioma: a matched-cohort analysis. Int J Radiat Oncol Biol Phys 85 (2): 315-20, 2013.
- Liu AK, Macy ME, Foreman NK: Bevacizumab as therapy for radiation necrosis in four children with pontine gliomas. Int J Radiat Oncol Biol Phys 75 (4): 1148-54, 2009.
- Freeman CR, Krischer JP, Sanford RA, et al.: Final results of a study of escalating doses of hyperfractionated radiotherapy in brain stem tumors in children: a Pediatric Oncology Group study. Int J Radiat Oncol Biol Phys 27 (2): 197-206, 1993.
- Izzuddeen Y, Gupta S, Haresh KP, et al.: Hypofractionated radiotherapy with temozolomide in diffuse intrinsic pontine gliomas: a randomized controlled trial. J Neurooncol 146 (1): 91-95, 2020.
- Zaghloul MS, Eldebawy E, Ahmed S, et al.: Hypofractionated conformal radiotherapy for pediatric diffuse intrinsic pontine glioma (DIPG): a randomized controlled trial. Radiother Oncol 111 (1): 35-40, 2014.
- Negretti L, Bouchireb K, Levy-Piedbois C, et al.: Hypofractionated radiotherapy in the treatment of diffuse intrinsic pontine glioma in children: a single institution's experience. J Neurooncol 104 (3): 773-7, 2011.
- Freeman CR, Kepner J, Kun LE, et al.: A detrimental effect of a combined chemotherapy-radiotherapy approach in children with diffuse intrinsic brain stem gliomas? Int J Radiat Oncol Biol Phys 47 (3): 561-4, 2000.
- Bradley KA, Zhou T, McNall-Knapp RY, et al.: Motexafin-gadolinium and involved field radiation therapy for intrinsic pontine glioma of childhood: a children's oncology group phase 2 study. Int J Radiat Oncol Biol Phys 85 (1): e55-60, 2013.
- Kestle J, Townsend JJ, Brockmeyer DL, et al.: Juvenile pilocytic astrocytoma of the brainstem in children. J Neurosurg 101 (1 Suppl): 1-6, 2004.
- Igboechi C, Vaddiparti A, Sorenson EP, et al.: Tectal plate gliomas: a review. Childs Nerv Syst 29 (10): 1827-33, 2013.
- Bilaniuk LT, Molloy PT, Zimmerman RA, et al.: Neurofibromatosis type 1: brain stem tumours. Neuroradiology 39 (9): 642-53, 1997.
- Molloy PT, Bilaniuk LT, Vaughan SN, et al.: Brainstem tumors in patients with neurofibromatosis type 1: a distinct clinical entity. Neurology 45 (10): 1897-902, 1995.
- Klimo P, Pai Panandiker AS, Thompson CJ, et al.: Management and outcome of focal low-grade brainstem tumors in pediatric patients: the St. Jude experience. J Neurosurg Pediatr 11 (3): 274-81, 2013.
- Ronghe M, Hargrave D, Bartels U, et al.: Vincristine and carboplatin chemotherapy for unresectable and/or recurrent low-grade astrocytoma of the brainstem. Pediatr Blood Cancer 55 (3): 471-7, 2010.
- Ruge MI, Kickingereder P, Simon T, et al.: Stereotactic iodine-125 brachytherapy for treatment of inoperable focal brainstem gliomas of WHO grades I and II: feasibility and long-term outcome. J Neurooncol 109 (2): 273-83, 2012.
Progressive / Recurrent Childhood Brain Stem Glioma Treatment
Treatment Options for Progressive Childhood Diffuse Intrinsic Pontine Gliomas (DIPGs)
Progression of the DIPG tumor is anticipated generally within 1 year of completing radiation therapy. Radiation-induced changes may occur a few months after the completion of radiation therapy and may mimic tumor progression (also known as pseudoprogression).[1]
In most cases, biopsy at the time of clinical or radiologic progression is neither necessary nor recommended. Biopsy may be considered for confirmation of relapse when treatment-related brain stem damage, which may be clinically indistinguishable from tumor recurrence, is in the differential diagnosis. Other tests, including positron emission tomography, magnetic resonance spectroscopy, and single-photon emission computed tomography, have not yet been shown to be reliable in distinguishing necrosis from tumor recurrence in previously irradiated DIPG patients.
When considering the efficacy of additional treatment, care needs to be taken to separate radiation-induced changes from progressive disease.
There is no standard treatment. Treatment options for progressive childhood DIPGs include the following:
- Radiation therapy.
Reirradiation has been shown to prolong survival and can be considered at progression in children who have had an initial response to radiation therapy.[2,3]
- A clinical trial of a novel therapeutic approach.
Patients or families who desire additional disease-directed therapy should consider entering trials of novel therapeutic approaches because no standard agents have demonstrated clinically significant activity.
Regardless of whether a decision is made to pursue disease-directed therapy at the time of progression, palliative care remains a central focus of management. This ensures that quality of life is maximized while attempting to reduce symptoms and stress related to the terminal illness.
Treatment options under clinical evaluation for progressive childhood DIPGs
Early-phase therapeutic trials may be available for selected patients. These trials may be available via the Children's Oncology Group (COG), the Pediatric Brain Tumor Consortium (PBTC), or other entities. Information about National Cancer Institute (NCI)–supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following are examples of national and/or institutional clinical trials that are currently being conducted:
- APEC1621 (NCT03155620) (Pediatric MATCH: Targeted Therapy Directed by Genetic Testing in Treating Pediatric Patients with Relapsed or Refractory Advanced Solid Tumors, Non-Hodgkin Lymphomas, or Histiocytic Disorders): NCI-COG Pediatric Molecular Analysis for Therapeutic Choice (MATCH), referred to as Pediatric MATCH, will match targeted agents with specific molecular changes identified in a patient's tumor (refractory or recurrent). Children and adolescents aged 1 to 21 years are eligible for the trial. Patients with a brain stem glioma may be enrolled if tissue is available from a diagnostic biopsy.
Patients with tumors that have molecular variants addressed by treatment arms included in the trial will be offered treatment on Pediatric MATCH. Additional information can be obtained on the NCI website and ClinicalTrials.gov website.
- PBTC-047 (NCT02717455) (Panobinostat in Treating Younger Patients With DIPG): This phase I trial studies the side effects and best dose of panobinostat in treating younger patients with DIPGs. Panobinostat may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. Stratum 1 treats patients with DIPGs that have returned or gotten worse (progressed). Stratum 2 treats patients with DIPGs that have not yet gotten worse.
- PBTC-051 (NCT03389802) (CD40 Agonistic Monoclonal Antibody APX005M in Treating Pediatric Patients With Recurrent or Refractory Brain Tumors): This phase I trial studies the side effects and best dose of APX005M in treating younger patients with primary malignant central nervous system tumors that are growing, spreading, or getting worse (progressive), or newly diagnosed DIPGs. APX005M can trigger activation of B cells, monocytes, and dendritic cells and stimulate cytokine release from lymphocytes and monocytes. APX005M can mediate a direct cytotoxic effect on CD40-positive tumor cells.
Treatment Options for Recurrent Childhood Focal Brain Stem Gliomas
At the time of tumor recurrence, a complete evaluation to determine the extent of the relapse may be indicated for selected focal lesions. Treatment considerations at the time of recurrence or progression are dependent on prior treatment.
Treatment options for recurrent childhood focal brain stem gliomas include the following:
- Surgery.
The need for surgical intervention must be individualized on the basis of the initial tumor type, the location within the brain stem, the length of time between initial treatment, the appearance of the mass lesion, and the clinical picture.[4]
- Radiation therapy.[5]
- Chemotherapy.
Chemotherapy with agents such as carboplatin and vincristine may be effective in children with recurrent focal exophytic gliomas.[6]
Treatment options under clinical evaluation for recurrent childhood focal brain stem gliomas
Early-phase therapeutic trials may be available for selected patients. These trials may be available via the COG, the PBTC, or other entities. Information about NCI-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- APEC1621 (NCT03155620) (Pediatric MATCH: Targeted Therapy Directed by Genetic Testing in Treating Pediatric Patients with Relapsed or Refractory Advanced Solid Tumors, Non-Hodgkin Lymphomas, or Histiocytic Disorders): NCI-COG Pediatric Molecular Analysis for Therapeutic Choice (MATCH), referred to as Pediatric MATCH, will match targeted agents with specific molecular changes identified in a patient's tumor (refractory or recurrent). Children and adolescents aged 1 to 21 years are eligible for the trial. Patients with a brain stem glioma may be enrolled if tissue is available from a diagnostic biopsy.
Patients with tumors that have molecular variants addressed by treatment arms included in the trial will be offered treatment on Pediatric MATCH. Additional information can be obtained on the NCI website and ClinicalTrials.gov website.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References:
- Carceller F, Fowkes LA, Khabra K, et al.: Pseudoprogression in children, adolescents and young adults with non-brainstem high grade glioma and diffuse intrinsic pontine glioma. J Neurooncol 129 (1): 109-21, 2016.
- Janssens GO, Gandola L, Bolle S, et al.: Survival benefit for patients with diffuse intrinsic pontine glioma (DIPG) undergoing re-irradiation at first progression: A matched-cohort analysis on behalf of the SIOP-E-HGG/DIPG working group. Eur J Cancer 73: 38-47, 2017.
- Lassaletta A, Strother D, Laperriere N, et al.: Reirradiation in patients with diffuse intrinsic pontine gliomas: The Canadian experience. Pediatr Blood Cancer 65 (6): e26988, 2018.
- Bowers DC, Krause TP, Aronson LJ, et al.: Second surgery for recurrent pilocytic astrocytoma in children. Pediatr Neurosurg 34 (5): 229-34, 2001.
- Huynh-Le MP, Walker AJ, Burger PC, et al.: Management of pediatric intracranial low-grade gliomas: long-term follow-up after radiation therapy. Childs Nerv Syst 32 (8): 1425-30, 2016.
- Ater JL, Zhou T, Holmes E, et al.: Randomized study of two chemotherapy regimens for treatment of low-grade glioma in young children: a report from the Children's Oncology Group. J Clin Oncol 30 (21): 2641-7, 2012.
Changes to This Summary (02 / 23 / 2022)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
Editorial changes were made to this summary.
This summary is written and maintained by the PDQ Pediatric Treatment Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® - NCI's Comprehensive Cancer Database pages.
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of childhood brain stem glioma. It is intended as a resource to inform and assist clinicians in the care of their patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the PDQ Pediatric Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewers for Childhood Brain Stem Glioma Treatment are:
- Kenneth J. Cohen, MD, MBA (Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins Hospital)
- Karen J. Marcus, MD, FACR (Dana-Farber Cancer Institute/Boston Children's Hospital)
- Roger J. Packer, MD (Children's National Hospital)
- D. Williams Parsons, MD, PhD
- Malcolm A. Smith, MD, PhD (National Cancer Institute)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Pediatric Treatment Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as "NCI's PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary]."
The preferred citation for this PDQ summary is:
PDQ® Pediatric Treatment Editorial Board. PDQ Childhood Brain Stem Glioma Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/brain/hp/child-glioma-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389253]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images.
Disclaimer
Based on the strength of the available evidence, treatment options may be described as either "standard" or "under clinical evaluation." These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website's Email Us.
Last Revised: 2022-02-23
Topic Contents
- General Information About Childhood Brain Stem Glioma
- Molecular Features of Childhood Brain Stem Glioma
- Stage Information for Childhood Brain Stem Glioma
- Treatment Option Overview for Childhood Brain Stem Glioma
- Newly Diagnosed Childhood Brain Stem Glioma Treatment
- Progressive / Recurrent Childhood Brain Stem Glioma Treatment
- Changes to This Summary (02 / 23 / 2022)
- About This PDQ Summary
This information does not replace the advice of a doctor. Healthwise, Incorporated, disclaims any warranty or liability for your use of this information. Your use of this information means that you agree to the Terms of Use. Learn how we develop our content.
